The value of CD19 directed CAR T cell therapy for relapsed, refractory B-ALL has been well established. Iterative and complementary clinical trials have identified subgroups with improved outcomes, including those with low disease burden at the time of treatment as well as those with prolonged CAR T cell in vivo persistence. PLAT-02 was a phase 1/2 study of SCRI-CAR19, a 2 nd generation murine CAR with 41BB co-stimulation. Phase 1 previously reported 1 year LFS, event-free (EFS) and overall survival (OS) of 55%, 51.2%, and 69.5%. Subjects with low CD19 antigen burden in the bone marrow (<15%) prior to lymphodepletion (LD) and those with a rapid contraction of CAR T cells between Day 10 and 14 (ratio ≥1.5) were at higher risk of early loss of persistence. Phase 2 of PLAT-02 was a multisite trial along with a single site pilot study, PLAT-03, to explore administration of exogenous CD19 antigen designed to boost CAR T cell engraftment in subjects at high risk of early loss of persistence.
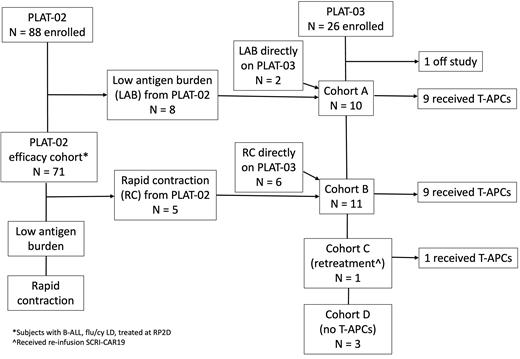
PLAT-02 (NCT02028455) enrolled subjects 1-26 years of age with relapsed, refractory CD19+ B-ALL. The primary efficacy cohort was defined as those who received fludarabine/cyclophosphamide (flu/cy) LD and were infused with CAR19. PLAT-03 (NCT03186118) was a pilot study of CD19t T-APCs (manufactured T cells with CD19 tag) administered at doses of 10x10 6/kg or flat dosing of 5x10 8 for subjects >25kg every 4 weeks up to 6 total doses. Cohort A included subjects with low CD19 antigen burden, and cohort B included those with rapid early contraction of CAR19.
88 subjects with B-ALL were enrolled on PLAT-02 with plans for treatment at the recommended phase 2 dose (RP2D) of 1x10 6 CAR+ T cells/kg. Eighty-six percent had at least one prior relapse and 42% had undergone prior hematopoietic cell transplant (HCT). The feasibility of manufacturing was 98%. 71 subjects were in the primary efficacy cohort with an 89% minimal residual disease negative complete remission (MRDneg CR) rate. EFS and OS probability estimates with 95% CI at 1 year following CAR19 were 0.65 (0.53, 0.75) and 0.76 (0.64, 0.84). LFS at 1 year from entering MRDneg CR was 0.71 (0.58, 0.81). Cytokine release syndrome (CRS) was grade (Gr) 1 (53%), Gr 2 (26%), Gr 3 (10%), and Gr 4 (3%). Neurotoxicity occurred in 53%: Gr 1 (24%), Gr 2 (13%), Gr 3 (15%), and Gr 5 (1%).
25 subjects were treated on PLAT-03, 10 as cohort A (10 received CAR19 on PLAT-03, 9 received T-APCs), and 11 as cohort B (5 received CAR19 on PLAT-02, 6 received CAR19 on PLAT-03, and 9 total received T-APCs). One was cohort C (retreatment) and 3 were cohort D (no T-APCs). A total of 72 infusions of T-APCs were administered, with no occurrence of CRS or neurotoxicity. Two subjects had grade 3 toxicities related to T-APCs, 1 febrile neutropenia and 1 infusion related reaction. EFS and OS probability estimates at 1 year following CAR19 were 0.67 (0.43, 0.83) and 0.95 (0.71, 0.99). Overall LFS at 1 year was 0.63 (0.38, 0.80); by cohort: cohort A, 0.44 (0.13, 0.72) and cohort B, 0.80 (0.41, 0.95).
Median duration of persistence, as determined by cumulative incidence using B cell aplasia (BCA) as a surrogate, among all subjects who received RP2D of CAR19 with flu/cy LD across PLAT-02 and PLAT-03, engrafted and achieved MRDneg CR, was 12.9 months (m). For subjects who did not receive T-APCs, the median persistence was 12.1m. Impact of antigen burden prior to LD was again observed; those with >15% CD19 antigen burden had persistence of >12.1m vs. 3.9m in the low antigen burden group. For subjects treated with T-APCs, the median persistence was 16m; 9.9m in cohort A and >16m in cohort B.
The improvement in LFS after SCRI-CAR19 in phase 2 compared to phase 1 is likely multi-factorial, with contributing factors including earlier treatment, uniform use of LD, enhanced persistence, and better allocation of patients who benefit from consolidative HCT. The use of T-APCs was well tolerated without recurrent CAR T cell mediated toxicity. Although not directly comparable, there did appear to be enhancement of CAR T cell persistence with the use of T-APCs in populations predicted to have inferior persistence, including those with low antigen burden. There was not a clear improvement of LFS in those who received T-APCs. However, subjects were not randomized to receive T-APCs and those enrolled on PLAT-03 were more heavily treated and more likely to have received a prior HCT. Further exploration of T-APCs is warranted to define and evaluate their impact on persistence and durable responses.
Disclosures
Agrawal:Y-mAbs Therapeutics: Membership on an entity's Board of Directors or advisory committees. Pulsipher:Vertex Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; CARGO Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; GentiBio: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Research Funding; Miltenyi Biotec: Research Funding. Jensen:Bristol-Myers Squibb: Patents & Royalties. Gardner:Bristol-Myers Squibb: Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal